Machinable, Larger-Scale, Self-Healing Refractory High-Entropy Alloys (RHEA) for Energy and Aerospace Applications
RHEAs are special materials made up of four or more elements that can withstand very high temperatures. These elements are often mixed in equal amounts. Because of the variety of elements and their sizes, RHEAs form unique structures that help them stay stable and strong, even when exposed to extreme conditions.
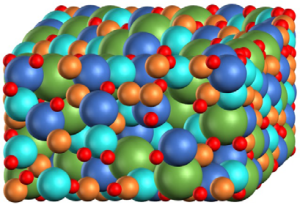
The Sandia team developed an additive manufacturing,
rapid prototyping, continuous improvement process
and software for manufacturing larger-scale, self-healing,
high-quality, machinable RHEAs for high temperature,
radiative, and corrosive environments.
Groundbreaking advancements in materials engineering were achieved with the development of an additive manufacturing, rapid-prototyping, continuous improvement process, as well as relevant research software. This enabled Sandia to manufacture larger scale, high-quality RHEAs with a high degree of machinability and harsh-environment resilience. Notably, the Sandia team manufactured a RHEA weighing a world record 7.72 lbs. and measuring 10.3” by 8.6” by 0.26”.
Low-cost Direct Air Capture of Carbon Dioxide with Clay Nano-Interlayers (LDAC3)

LDAC3 is an innovative technology designed to efficiently capture carbon dioxide (CO2) from the air using a natural and affordable material. This system leverages two key concepts: the ability to dissolve gases more effectively in tiny spaces and the rapid movement of water through tiny channels within the material.
At its core, the system uses a membrane made from a common clay, such as smectite, which selectively attracts CO2 and water vapor while filtering out other gases like nitrogen and oxygen. Remarkably, the presence of water in the clay’s nano-sized layers enhances CO2 absorption by over 55 times. The captured CO2 is then drawn through the membrane by creating a difference in humidity on either side, allowing for efficient collection. By carefully managing this humidity difference, the energy needed for capturing CO2 is significantly reduced, making this technology much more cost-effective than existing methods, plus the entire process is environmentally benign and simple to use, with no chemicals involved.
Sandia’s Laboratory Directed Research and Development office supported this research.

