Acoustic Wave Biosensors

A particular class of devices known as shear horizontal surface (SH-SAW) sensors is well-suited for the detection of biological agents in liquid environments. These devices have the dual advantages of high sensitivity (down to picograms/cm2) and high specificity conferred by biological receptors such as antibodies, peptides, and nucleic acids. Sandia has demonstrated the detection of bacteria, viral particles, and proteins with these sensors. Handheld biodetection systems incorporating these microsensors are under development.
Sample Preparation

Sandia has developed a miniature acoustic lysing system that rapidly releases nucleic acids and proteins from cellular samples, enabling more efficient DNA sample preparation. The technology provides a fast and reagentless method to produce a continuous source of lysate without harsh chemical agents. The present system uses plastic cartridges to couple the microchannel lysing regions to a standalone acoustic actuator array. The disposable microchannel cartridges are fabricated using layers of mylar and acrylic, which can be built-up to create complex 3D structures.
When compared to commercial lysing instruments, the Sandia system provides higher efficiency, takes less time, consumes less power, and occupies a much smaller footprint. Sandia has further developed a DNA extraction method that uses magnetic-core beads with an electrostatic surface to bind and release DNA. This module integrates into the lysing system, providing polymerase chain reaction (PCR) quality DNA.
Bead-Based Microsensors
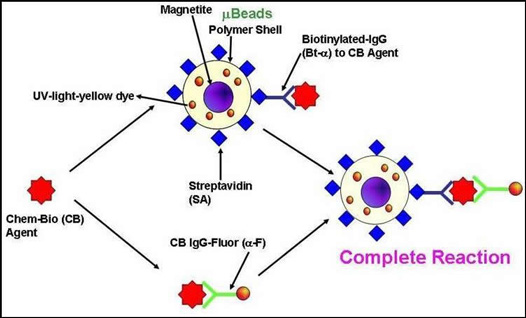
Sandia is developing a new battlefield adaptable agent detection platform capable of sensing multiple threat agents (chemical, biological and nuclear) simultaneously in diverse media, as well as a performance model of the detection platform. Magnetic microspheres impregnated with chromophoric dyes or Quantum Dots (QD) are used to develop sensitive tests for chem-bio-threat agents (CB-Agent). A variety of magnetic microspheres (µbeads) may be impregnated with QD or dyes for barcoding. The surfaces of the microspheres are functionalized with streptavidin protein. Biotinylated antibodies to various chem-bio-threat agents may then be stably anchored to the µbeads. The ratio of the optical signature (fluorescence, chemiluminescence or absorption) in the presence and absence of the chemical or biological agent is an indicator for a particular agent.
Electrochemical Biosensor Arrays
The reliable and definitive detection of multiple biowarfare agents on a single robust platform would be a significant asset for the defense of our nation and the safeguarding of warfighters. Multiple signature based biosensors can meet this need as they not only allow for multianalyte detection, but also substantially increase confidence in the sensor output as whole cell, genomic, and proteomic data can be interrogated for each target analyte. Sandia has developed a method that allows for controlled and selective immobilization of biorecognition elements onto electrodes, allowing simultaneous multianalyte detection of DNA and proteins.
ElectroNeedles
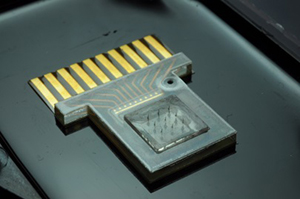
In medical diagnostics, quick, precise, and accurate results are desirable for point of care use. Harvesting of carbohydrate fuels, glucose, from humans involves the extraction of interstitial fluid (ISF) or blood. Sandia National Laboratories’ ElectroNeedles has proven a platform capable of detecting up to 50 individual analytes real-time and in vivo, without causing pain to the patient.
Microneedles provide an ideal platform to painlessly extract ISF. Using hollow microneedles, Sandia teams have withdrawn glucose from a donor solution (10.1 mM glucose in physiological buffer), using a Franz diffusion cell and porcine skin. ElectroNeedles could be used to detect a wide range of analytes including glucose, enzymes, proteins, antibodies, whole cells, and toxins.
Cell-Based Biosensors
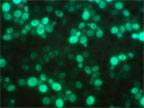
Sandia has developed genetically tailored cells and bio-compatible self-assembly approaches to enable whole cell sensors in patterned fluidic architectures for chemical and biological sensing. Combining genetics and molecular biology techniques, Sandia designed three reporter/promoter constructs that are integrated directly into yeast chromosomes that produce green, cyan, and red fluorescent protein in response to cholera toxin exposure.
Nanostructure-Induced Fluorescence Detection
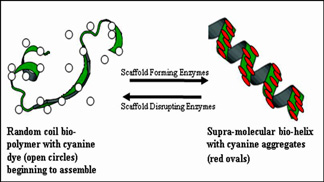
Geometries of nature are the subject of intense interest to chemists, biologists, physicists and mathematicians. Examples of natural geometries are found in living organisms (sea shells, for example) and among molecular components. Supra-molecular self-assembled aggregates yield interesting geometries through a collection of molecules held together by non-covalent bonds such as electrostatic forces, hydrogen bonding, or hydrophobic interactions, to provide homogeneous or heterogeneous assemblies.
Certain cyanine dyes form molecular aggregates that are either the J- or H-type depending on their chemistry and sample milieu. H-aggregates have a blue-shifted absorption band, relative to the monomeric dye absorption wavelength. J-aggregates have a narrower, red-shifted absorption band, compared to the monomer; J-aggregates display sharp, intense fluorescence emission. Spectral properties of J- and H-aggregates make them attractive candidates for developing a variety of chem-bio-sensing applications.
Photonic Biosensors
Sandia combined bio-assay technologies with its own state-of-art photonics technologies to develop a compact, planar, arrayable, optically based biosensor with unprecedented sensitivity. The sensor devices, fabricated in Sandia’s Microelectronics Development Laboratory, employ guided wave structures enhanced by the addition of ring-resonators. Overall sensitivity is further enhanced by using gold or quantum dot nanoparticles as the attached optical reporter tags to amplify the optical response, with a goal of being able to detect as little as one bound DNA strand.
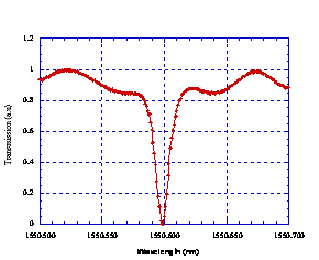
Figure: Evanescent Microresonant Ring with a Q = 240,000. Diameter was 400 µm, cross-section was 1.2 µm x 0.2µm
Electrocatalytic Nanoparticles
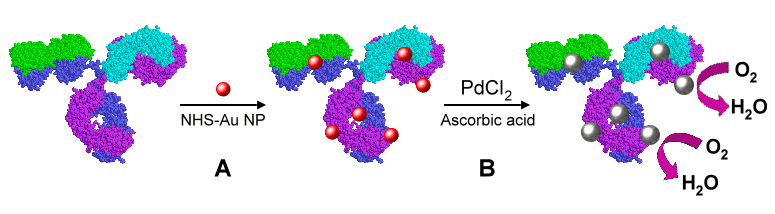
A significant disadvantage of many biosensors is the requirement of labels or reagents for sensitive and specific biological detection. Sandia has shown that utilization of electrocatalytic nanoparticles allows for reagent-less and highly sensitive protein detection. Sandia is conducting work on electrocatalytic nanoparticles, focusing on the development of reagent-less and label-free methods for biological detection using the unique properties of nanoparticles.
Figure: Approach Sandia developed for electrochemical immunoassay sensing in which Pd NPs can be loaded onto an anti-TNF-a antibody to create an electrocatalytic antibody. Gold particles are first covalently linked to the antibody (step A). These gold nanoparticles then act as a seed for growth of a palladium shell (step B).
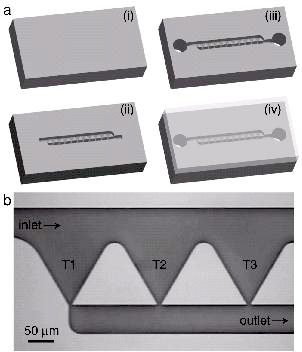
MicroFluidics
Monitoring the immune response in single host cells challenged with pathogens is required in order to understand the stochasticity of immune response in a population of cells. Sandia has developed a microfluidic platform for optical interrogation of an array of single host cells. Additionally, Sandia has developed an electrofluidic platform for impedimetric interrogation of single host cells during pathogenic challenges.
Figure 1: (left) Schematic of the (a) SCA chip fabrication and (b) optical micrograph of the chip. (right) (a-d) Macrophage held in a trap during an exposure to LPS. (e) Fluorescence intensity across the cell and (f) the ratio of nuclear to cytoplasm fluorescence as a function of time.

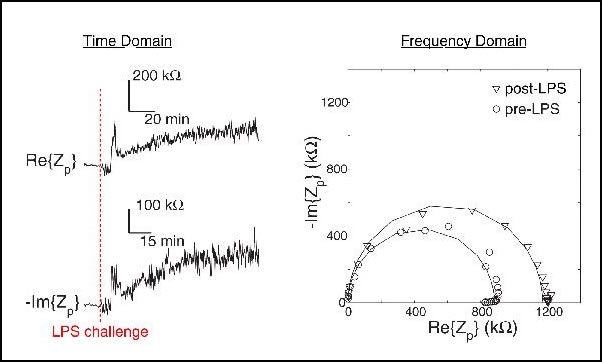
Figure 3: (left) Real and imaginary components of the impedance across a single macrophage as a function of time during an LPS challenge. (right) Nyquist plot of the macrophage before and after LPS exposure.