A portable chemical sensor system the size of a soccer ball being developed by Sandia scientists promises a new way of detecting and identifying even the smallest traces of explosives under water — whether in a rice paddy or deep under the ocean.

The system takes a sample of liquid drawn from water surrounding submerged objects that contain explosives, extracts the molecules of interest on a fiber, desorbs the molecules from the fiber into an Ion Mobility Spectrometer (IMS), and identifies the explosive based on chemical signatures of the material.
"This system fills a unique niche," says Ron Woodfin (2552), project manager. "Unlike the commonly used anomaly detectors, such as metal detectors or ground-penetrating radar, the IMS analyzes the material’s actual molecular makeup to identify the explosive type. Its best role is not as a search device, but as a classifier or identifier."
Miniaturization efforts
At the heart of the system is a new Sandia-developed concentration technology that gathers samples on a fiber and concentrates it thousands of times, making the levels high enough to analyze.
Ron assembled a team with Phil Rodacy (2552), serving as the technical lead, and consisting of Bill B. Chambers (5336), Pam Walker (2552), Steve Reber (2552), and Jim Phelan (6131) to develop the sensor system. Not only did they devise the new concentration technology, but they also miniaturized the IMS, reducing it from a commercially made 30-pound shoebox size device to a five-pound unit that fits in a person’s hand. When complete, the entire sensor system — including the IMS, concentrator, computer, display, and batteries — will weigh no more than 20 pounds and be the size of a soccer ball. The small size makes it practical and economical to use.
The IMS was selected to be part of the sensor system because it offers a balance of sensitivity and specificity in identifying and isolating explosive molecules. It is similar to technology found in the Sandia-developed explosives-detection portal (Lab News, Sept. 12, 1997), which checks airline passengers to see if they are carrying explosives — only smaller.
Through a series of experiments performed at the US Navy facility at San Clemente Island off the coast of San Diego in 1996-98, another in the waters near Panama City, Fla., last year, and most recently this August and September in Halifax, Nova Scotia, the researchers showed that the detection system works. Samples of water close to known sites with explosives were successfully collected, concentrated, and analyzed.
"Now that we have proven that the technology works we need to get it even smaller and able to function under water," Ron says.
The system works because of several factors. First is the phenomenon that all ammunitions or unexploded ordnance items that contain explosives emit molecules of explosive chemicals contained inside them. If they are submerged in water, the molecules can be found downstream from the explosives. If they are buried in soil, traces can be located in nearby dirt.
The second factor that contributes to the successful sensor system is that a certain type of polymer fiber (polydimethysiloxane/divinylbenzene copolymer) attracts specific types of explosive molecules in cool temperatures.
The third is that the molecules lose their attraction to the fiber when it is heated slightly, causing them to rapidly desorb.
Taking all this into account, the researchers developed a method where they insert the polymer fiber into a syringe used to collect water samples near underwater explosive devices. The near-dry fiber containing the molecules is then removed from the collecting device and placed in the IMS where it is heated slightly. The molecules jump off the fiber. By measuring the rate at which the molecules transit the IMS the researchers determine the identity of the explosive or if it is an explosive at all.
Currently the chemical sensor system can only work outside water, but the researchers are developing a new waterproof packaging so that it will function under water.
Sandia recently signed a three-year agreement with Lockheed Martin to develop an underwater sensor system that would be attached to an unmanned underwater vehicle. The vehicle, being built by Lockheed Martin, would use sonar to find a suspicious object, like an unexploded ordnance. It would determine the location of the current downstream from the object and move to that point where the sensor system would take a sample and automatically run an analysis.
The sensor system has successfully analyzed soil field samples in the laboratory at Sandia’s Explosive Components Facility, but, Ron says, it is much more effective on samples that have the explosives dissolved in water, and that’s why they are presently concentrating on that area of research.
Currently, the predominant way of seeking out explosives both on the ground and in water is by using anomaly detectors that identify objects not expected in the environment. A common one is the metal detector, which locates anything with metal — most of which are not ordnance.
"The IMS system exploits a different principle than the anomaly technique," Phil says. "It looks for actual explosive materials, which eliminates the numbers of false alarms. Either it’s an explosive or it’s not."
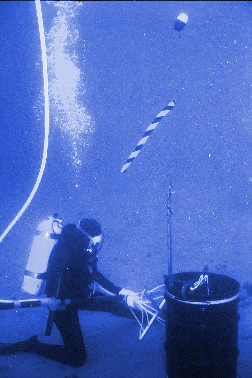
Initial tests of the sensor system were done in 1996 through 1998 at San Clemente Island. The researchers prepared simulated explosive devices using oil drums doped with trace amounts of explosives, submerged them, and sent divers in to collect water near the test area. They were able to detect concentrations of the explosives at distances of more than 40 feet from the source. These initial tests were where the concentration concept was developed.
In a test last year near Panama City, Fla., the researchers submerged actual miliatry hardware containing explosives and collected water near the site. Once again the system was successful in identifying explosive material.
Most recently, in August and September, the researchers went to Halifax, Nova Scotia, where they tested waters in the Bedford Basin near two ammunition-laden ships, the Claire Lilly and the Trongate, that sank in World War II. From the watery graves of these two ships the researchers obtained samples and used the portable chemical sensor system to determine that traces of explosives existed, primarily TNT, cordite, and other materials used 50 years ago.
The earlier San Clemente and Panama City tests were sponsored by Sandia, the Office of Naval Research, and the Department of Defense, Office of Munitions. The Nova Scotia venture was sponsored by the Office of Naval Research and hosted by the Defence Research Establishment Atlantic (part of the Canadian Department of National Defence) with help from the Canadian Fleet Diving Unit.
Phil anticipates that as the sensor research matures with the Lockheed Martin agreement, the technology will rapidly move from the prototype stage to the development stage.
"As we further develop this process, this IMS-based sensor has potential to rapidly and accurately classify explosive ordnance items, doing underwater what demining dogs [canines that sniff out explosives] do today on land, only using electronic and chemical technology," Phil says.