Helium nanobubbles in metals
Helium can accumulate in metals in radiation environments. It is generally very insoluble, and can precipitate to form nanometer-scale helium bubbles. We are interested in understanding how the bubbles form and evolve, and how they affect macroscopic material properties.
- Taylor CA, DB Robinson, JD Sugar, E Lang, CM Barr, Y Wang, CS Snow, KM Hattar. (2022). Microstructural Effects of High Dose Helium Implantation in ErD2. Materialia 22:101280.
- Taylor CA, JD Sugar, DB Robinson, NC Bartelt, RB Sills, KM Hattar. (2020). Using In-situ TEM Helium Implantation and Annealing to Study Cavity Nucleation and Growth. JOM 72:2032.
- Dennett CA, RC Choens, CA Taylor, NM Heckman, MD Ingraham, DB Robinson, BL Boyce, MP Short, K Hattar. (2020). Listening to radiation damage in situ: passive and active acoustic techniques. JOM 72:197.
- Taylor, CA, S Briggs, G Greaves, A Monterrosa, E Aradi, JD Sugar, DB Robinson, K Hattar, J Hinks. (2019). Investigating Helium Bubble Nucleation and Growth through Simultaneous In-situ Cryogenic, Ion Implantation, and Environmental Transmission Electron Microscopy. MDPI Materials 12:2618.
- Catarineu NR, NC Bartelt, JD Sugar, S Vitale, KL Shanahan, DB Robinson. 3D Maps of Helium Nanobubbles to Probe the Mechanisms of Bubble Nucleation and Growth. J. Phys. Chem. C 123:19142.
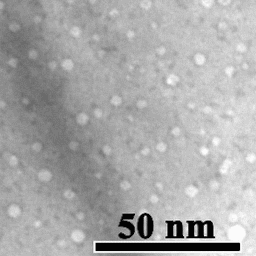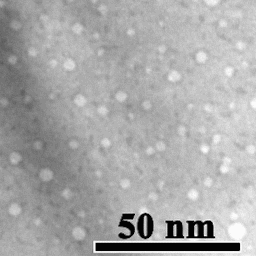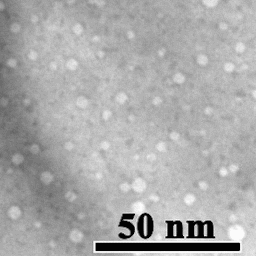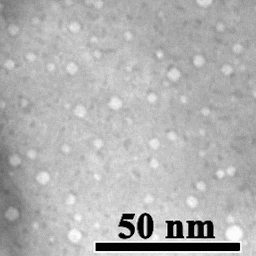
Motion of helium nanobubbles in palladium, formed by helium ion implantation, at elevated temperature in a transmission electron microscope.
Hydrogen storage and transport in nanoporous palladium alloys
Palladium is a high-performance storage material for hydrogen isotopes, and enhanced properties are being discovered when the material has nanoscale structure. If all of the hydrogen is within a few nanometers of an interface, we expect charge and discharge rates to be faster and hysteresis to be lower. When tritium is used, it should be easy for decay products to escape without damaging the material. We are making nanoporous palladium powders and using them to test these hypotheses.
- Robinson DB, M Salloum. (2023). Comparison of Designs of Hydrogen Isotope Separation Columns by Numerical Modeling. Ind. Eng. Chem. Res. 62:3647.
- Cappillino PJ, DB Robinson. (2018). Porous metals from sintering of nanoparticles. US Patent 9981313 B1.
- Salloum, M, DB Robinson. (2018). A Numerical Model of Exchange Chromatography Through 3D Lattice Structures. AIChE Journal 64:1874.
- Robinson, DB. (2017). Compact Determination of Hydrogen Isotopes. Fusion Science and Technology 71:363.
- Robinson, DB, W Luo, TY Cai, KD Stewart. (2015). Metal hydride differential scanning calorimetry as an approach to compositional determination of mixtures of hydrogen isotopologues and helium. International Journal of Hydrogen Energy 40:14257.
- Salloum, M, SC James, DB Robinson. (2015). Effects of Surface Thermodynamics on Hydrogen Isotope Exchange Kinetics in Palladium: Particle and Flow Models. Chemical Engineering Science 122:474.
- Jones, CG, PJ Cappillino, V Stavila, DB Robinson. (2014). Control of both particle and pore size in nanoporous Pd powders. Powder Technology, 267:95.
- Robinson, DB. (2014). Hydrogen Isotope Exchange in a Metal Hydride Tube. Sandia Report SAND2014-17174 published at osti.gov.
- Shugard, AD, DB Robinson. (2014). A Simple Model of Gas Flow in a Porous Powder Compact. Sandia Report SAND2014-2858 published at osti.gov.
- Parent, LR, DB Robinson, PJ Cappillino, RJ Hartnett, P Abellan, JE Evans, ND Browning, I Arslan. (2014). In Situ Observation of Directed Nanoparticle Aggregation During the Synthesis of Ordered Nanoporous Metal in Soft Templates. Chemistry of Materials 26:1426.
- Cappillino, PJ, KM Hattar, BG Clark, RJ Hartnett, V Stavila, MA Hekmaty, BW Jacobs, DB Robinson. (2012). Synthesis of mesoporous palladium with tunable porosity and demonstration of its thermal stability by in situ heating and environmental transmission electron microscopy. Journal of Materials Chemistry A 1:602.
- Cappillino, PJ, MA Hekmaty, BW Jacobs, JD Sugar, V Stavila, PG Kotula, JM Chames, NY Yang, DB Robinson. (2012). Nanoporous Pd Alloys with Compositionally Tunable Hydrogen Storage Properties Prepared by Nanoparticle Consolidation. Journal of Materials Chemistry 22:14103.
- Parent, LR, DB Robinson, TJ Woehl, WD Ristenpart, JE Evans, ND Browning, I Arslan. (2012). Direct in Situ Observation of Nanoparticle Synthesis in a Liquid Crystal Surfactant Template. ACS Nano 6:3589.
- Ong, MD, BW Jacobs, JD Sugar, ME Grass, Z Liu, GM Buffleben, WM Clift, ME Langham, PJ Cappillino, DB Robinson. (2012). Effect of Rhodium Distribution on Thermal Stability of Nanoporous Palladium–Rhodium Powders. Chemistry of Materials 24:996.
- Klein, MP, BW Jacobs, MD Ong, SJ Fares, DB Robinson, V Stavila, GJ Wagner, I Arslan. (2011). 3-D Pore Evolution of Nanoporous Metal Particles for Energy Storage. Journal of the American Chemical Society 133:9144.
- Robinson, DB, ME Langham, SJ Fares, MD Ong, BW Jacobs, WM Clift, JK Murton, RP Hjelm, MS Kent. (2010). Thermally stable nanoporous palladium alloy powders by hydrogen reduction in surfactant templates. International Journal of Hydrogen Energy 35:5423.
- Robinson, DB, SJ Fares, MD Ong, I Arslan, ME Langham, KL Tran, WM Clift. (2009). Scalable synthesis of nanoporous palladium powders. International Journal of Hydrogen Energy 34:5585.
- Robinson, DB, SJ Fares. (2008). Bulk synthesis of nanoporous palladium and platinum powders. US Patent 8157886.
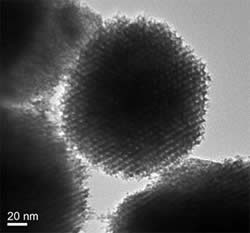
Transmission electron micrograph of nanoporous rhodium particle.
Ion transport and storage in nanoporous metal electrodes
When a nanoporous metal is soaked in a salty liquid, there can be enough surface area to remove bulk amounts of salt from the liquid through electrostatic and chemical adsorption. This can change how quickly ions move through pores. We have found ways to study and manipulate this effect, and are using it to design and build energy storage devices that can charge and discharge at rates near physical limits. In collaboration with Prof. Roger Narayan and his students at the joint biomedical engineering departments of North Carolina State University and the University of North Carolina, we have used these electrodes to controllably store and deliver high-value cargo such as therapeutic agents.
- Robinson, DB. (2019). 3D-Printed Apparatus for Efficient Fluid-Solid Contact. David B. Robinson, US Patent 10493693 B1.
- Robinson, DB, CG Jones. (2019). Electroless Deposition of Metal on 3D-Printed Polymeric Structures. US Patent 10494721 B1.
- Jones, CG, BE Mills, RK Nishimoto, DB Robinson. (2017). Electroless Deposition of Palladium on Macroscopic 3D-Printed Polymers with Dense Microlattice Architectures for Development of Multifunctional Composite Materials. Journal of the Electrochemical Society 164:D867.
- Martin, TM, DB Robinson, RJ Narayan. (2014). Nanoporous gold for biomedical applications: structure, properties, and applications. Chapter 7 (p. 148-162) in Precious Materials for Biomedical Applications. N. Baltzer and T. Copponnex, ed. Cambridge: Woodhead Publishing Ltd, 2014. ISBN 978 0 85709 233 5. (Republication of 2012 chapter below.)
- Chae, WS, H Yu, SK Ham, MJ Lee, JS Jung, DB Robinson. (2013). Bimodal porous gold opals for molecular sensing. Electronic Materials Letters 9:783.
- Martin, TM, DB Robinson, RJ Narayan. (2012). Nanoporous gold for biomedical applications: structure, properties and applications. Chapter 4 (p. 68-83) in Nanomedicine: Technologies and applications. T.J. Webster, ed. Cambridge: Woodhead Publishing Ltd, Oct 2012. ISBN 0 85709 233 2.
- Chae, WS, DV Gough, SK Ham, DB Robinson, PV Braun. (2012). Effect of Ordered Intermediate Porosity on Ion Transport in Hierarchically Nanoporous Electrodes. ACS Applied Materials and Interfaces 4:3973.
- Robinson, DB, CAM Wu, BW Jacobs. (2010). Effect of salt depletion on charging dynamics in nanoporous electrodes. Journal of The Electrochemical Society 157:A912.
- Robinson, DB, CAM Wu, MD Ong, BW Jacobs, BE Pierson. (2010). Effect of Electrolyte and Adsorbates on Charging Rates in Mesoporous Gold Electrodes. Langmuir 26:6797.
- Robinson, DB. (2010). Optimization of power and energy densities in supercapacitors. Journal of Power Sources 195:3748.
- Gittard, SD, BE Pierson, CM Ha, CAM Wu, RJ Narayan, DB Robinson. (2010). Supercapacitive transport of pharmacologic agents using nanoporous gold electrodes. Biotechnology Journal 5:192.
- Robinson, DB, SD Gittard, CAM Wu, CM Ha, RJ Narayan. (2010). Electrically modulated drug delivery using nanoporous electrodes. Micro- and Nanoscale Processing of Biomaterials Materials Research Society Symposium Proceedings 1239-VV08-03. Edited by R Narayan, S Jayasinghe, S Jin, W Mullins, D Shi.
Nanoporous gold opals with porosity on two length scales. Cover design by Sandia’s Vicente Garcia. Adapted with permission from ACS Applied Materials and Interfaces. © 2012 American Chemical Society.
Solution-phase deposition of multilayer films
We are expanding the capabilities of electrochemical atomic layer deposition (PDF, 586 KB) and similar techniques to create high-performance materials for hydrogen storage, as well as thermoelectric cooling and power generation.
- Gurung S, DB Robinson, PJ Cappillino. (2020). Palladium-Coated Platinum Powders with Tunable, Nanostructured Surfaces for Applications in Catalysis. ACS Applied Nano Materials 3:530.
- Benson, DM, CF Tsang, JD Sugar, K Jagannathan, DB Robinson, F El Gabaly, PJ Cappillino, JL Stickney. (2017). Enhanced Kinetics of Electrochemical Hydrogen Uptake and Release on palladium powders modified by Electrochemical Atomic Layer Deposition. ACS Applied Materials and Interfaces 9:18338.
- Jagannathan, K, DM Benson, DB Robinson, JL Stickney. (2016). Hydrogen Sorption Kinetics on Bare and Platinum-Modified Palladium Nanofilms, Grown by Electrochemical Atomic Layer Deposition (E-ALD). Journal of the Electrochemical Society 163:D3047.
- Cappillino, PJ, JD Sugar, F El Gabaly, TY Cai, Z Liu, JL Stickney, DB Robinson. (2014). Atomic-layer electroless deposition: a scalable approach to surface-modified metal powders. Langmuir 30:4820.
- Sheridan, LB, VM Yates, DM Benson, JL Stickney, DB Robinson. (2014). Hydrogen sorption properties of bare and Rh-modified Pd nanofilms grown via surface-limited redox replacement (SLRR) reactions. Electrochimica Acta 128:400.
- Robinson DB, PJ Cappillino, LB Sheridan, JL Stickney, DM Benson. (2013). Electroless atomic layer deposition. US Patent 9803285.
- Sheridan, LB, YG Kim, K Jagannathan, BR Perdue, JL Stickney, DB Robinson. (2013). Hydrogen Adsorption, Absorption, and Desorption at Palladium Nanofilms formed on Au(111) by Electrochemical Atomic Layer Deposition (E-ALD): Studies using Voltammetry and In Situ Scanning Tunneling Microscopy. The Journal of Physical Chemistry C 117:15728.
- Sheridan, LB, DK Gebregziabiher, JL Stickney, DB Robinson. (2013). Formation of Palladium Nanofilms Using Electrochemical Atomic Layer Deposition (E-ALD) with Chloride Complexation. Langmuir 29:1592.
- Lensch-Falk, JL, DO Banga, P Hopkins, DB Robinson, V Stavila, P. Sharma, DL Medlin. (2012). Electrodeposition and characterization of nanocrystalline antimony telluride thin films. Thin Solid Films 520:6109.
- Sheridan, LB, J Czerniawski, N Jayaraju, DK Gebregziabiher, JL Stickney, DB Robinson, MP Soriaga. (2012). Electrochemical Atomic Layer Deposition (E-ALD) of Palladium Nanofilms by Surface Limited Redox Replacement (SLRR), with EDTA Complexation. Electrocatalysis 3:96.
- Banga, D, JL Lensch-Falk, DL Medlin, V Stavila, NYC Yang, DB Robinson, PA Sharma. (2012). Periodic Modulation of Sb Stoichiometry in Bi2Te3/Bi2–xSbxTe3 Multilayers Using Pulsed Electrodeposition. Crystal Growth and Design 12:1347.
Porous materials for diverse energy applications
We have applied our skills developed in the above projects to benefit other energy materials projects at Sandia.
- Salloum M, DB Robinson. (2022). Optimization of Flow in Additively Manufactured Porous Columns with Graded Permeability. AIChE Journal 68(9):e17756.
- Reyes-Gil, KR, ZD Stephens, V Stavila, DB Robinson. (2015). Composite WO3/TiO2 nanostructures for high electrochromic activity. ACS Applied Materials & Interfaces 7:2202.
- Shugard, AD, GM Buffleben, TA Johnson, DB Robinson. (2014). Isotope Exchange between Gaseous Hydrogen and Uranium Hydride Powder. Journal of Nuclear Matererials 447:304.
- Reyes-Gil KR, DB Robinson (2013). WO3-Enhanced TiO2 Nanotube Photoanodes for Solar Water Splitting with Simultaneous Wastewater Treatment. ACS Applied Materials and Interfaces 5:12400.
- Stavila V, DB Robinson. MA Hekmaty, R Nishimoto, DL Medlin, S Zhu, TM Tritt, PA Sharma. (2013). Wet-Chemical Synthesis and Consolidation of Stoichiometric Bismuth Telluride Nanoparticles for Improving the Thermoelectric Figure-of-Merit. ACS Applied Materials and Interfaces 5:6678.
- Nenoff TM, BW Jacobs, DB Robinson, PP Provencio, JY Huang, S Ferreira, DJ Hanson. (2011). Synthesis and Low Temperature In Situ Sintering of Uranium Oxide Nanoparticles. Chemistry of Materials 23:5185.
Electron transfer kinetics at electrodes
In graduate school, I used well defined organic monolayers to study how charge crosses the boundary between an ion-conducting phase (like salty water) and an electron- or hole-conducting phase such as a metal. The process involves rearrangement of atoms into a favorable configuration, and then tunneling of a charge carrier through the monolayer. We are able to measure and control both of these steps.
- Robinson, DB, CED Chidsey. (2002). Submicrosecond Electron Transfer to Monolayer-Bound Redox Species on Gold Electrodes at Large Overpotentials. The Journal of Physical Chemistry B 106:10706.
- Cheng, J, DB Robinson, RL Cicero, T Eberspacher, CJ Barrelet, CED Chidsey. (2001). Distance Dependence of the Electron-Transfer Rate Across Covalently-Bonded Monolayers on Silicon. The Journal of Physical Chemistry B 105:10900.
- Barrelet, CJ, DB Robinson, TP Hunt, J Cheng, CF Quate, CED Chidsey. (2001). Surface Characterization and Electrochemical Properties of Alkyl, Fluorinated Alkyl and Alkoxy Monolayers on Silicon. Langmuir 17:3460.
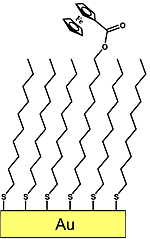
Charge transport through metal-organic-metal structures
Organic material is normally thought of as insulating, but on a scale of nanometers, appreciable amounts of charge can travel through it, and the process is strongly dependent on the structure of the material. The phenomenon is crucial to the use of energy by living things, and is of technological interest for energy harvesting and information processing. With various collaborators, I have made contributions to the box of tools and techniques necessary to take advantage of this.
- Robinson, DB, JR Funamura, AA Talin, RJ Anderson. (2007). Spectroscopic observation of chemical change during molecular electronics experiments. Applied Physics Letters 90:083119.
- Morpurgo, AF, CM Marcus, DB Robinson. (1999). Controlled Fabrication of Metallic Electrodes with Atomic Separation. Applied Physics Letters 74:2084.
- Wilson, TMS, DA Chinn, DB Robinson, FP Doty. (2008). Postpolymerization alignment of bulk conjugated polymers. Applied Physics Letters 93:143304.
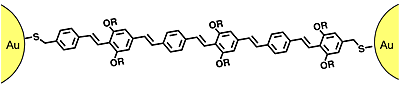
Single- or several-molecule biological binding assays using nanoparticles
In postdoctoral work before Sandia, I synthesized magnetic nanoparticles that are stable in biological environments, and attached DNA segments to them. I also worked to stabilize and functionalize microfabricated spin valve sensors that can detect small numbers of them. At Sandia, in collaboration with Lawrence Berkeley Lab’s Molecular Foundry, we have developed new small molecules for stabilizing nanoparticles in the salty conditions typically found in biology.
- Bogdanov, B, XN Zhao, DB Robinson, JH Ren. (2014). Electron capture dissociation tandem mass spectrometry studies of doubly protonated and mixed protonated-sodiated peptoids. Journal of the American Society for Mass Spectrometry 25:1202.
- Robinson, DB, GM Buffleben, ME Langham, RN Zuckermann. (2011). Stabilization of Nanoparticles under Biological Assembly Conditions Using Peptoids. Peptide Science 96:669.
- Robinson, DB, GM Buffleben, RN Zuckermann. Materials and Methods for Stabilizing Nanoparticles in Salt Solutions. US Patent 8461300.
- Robinson, DB, GM Buffleben, MS Kent, RN Zuckermann. (2011). Artificial polymers mimic bacteriophage capsid proteins to protect and functionalize nucleic acid structures. Chapter 3 in Amphiphiles: Molecular Assembly and Applications, R Nagarajan, ed., American Chemical Society Symposium Series.
- Morishetti, KK, SC Russell, XN Zhao, DB Robinson, JH Ren. (2011). Tandem Mass Spectrometry Studies of Protonated and Alkali Metalated Peptoids: Enhanced Sequence Coverage by Metal Cation Addition. International Journal of Mass Spectrometry 308:98.
- Robinson, DB, HH Persson, H Zeng, G Li, N Pourmand, S Sun, SX Wang. (2005). DNA-functionalized MFe2O4 (M = Fe, Co, or Mn) nanoparticles and their hybridization to DNA-functionalized surfaces. Langmuir 21:3096.
- Sun, S, H Zeng, DB Robinson, S Raoux, PM Rice, SX Wang, G Li. (2003). Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. Journal of the American Chemical Society 126:273. (In top 10 most cited papers in chemistry during summer of 2005, Science Watch, Nov–Dec 2005.)
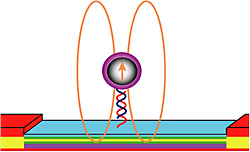
Strong biomimetic shell materials
Sandia’s interest in biofuels led us to study how diatoms (algae) grow the glass capsules that serve as their skeletons. For Sandia’s efforts to make compact bioanalytical instruments, we made tough polymer skeletons that helped with handling of organic capsules used to shuttle materials in microfluidic devices.
- Robinson, DB, JL Rognlien, CA Bauer, BA Simmons. (2007). Dependence of Amine-Accelerated Silicate Condensation on Amine Structure. Journal of Materials Chemistry 17:2113.
- Bauer, CA, DB Robinson, BA Simmons. (2007). Silica Particle Formation in Confined Environments via Bioinspired Polyamine Catalysts at Near-Neutral pH. Small 3:58.
- Robinson, DB, ES Lee, Z Iqbal, JL Rognlien, RV Davalos. (2007). Reinforced Vesicles Withstand Rigors of Microfluidic Electroporation. Sensors and Actuators B: Chemical 125:337.
- Lee, ES, DB Robinson, JL Rognlien, CK Harnett, BA Simmons, CRB Ellis, RV Davalos. (2006). Microfluidic electroporation of robust 10-µm vesicles for manipulation of picoliter volumes. Bioelectrochemistry 69:117.
Open-source research tools
- Sugar, JD, AW Cummings, BW Jacobs, DB Robinson. (2014). A Free Matlab Script for Spatial Drift Correction. Microscopy Today 22:40. Also archived at osti.gov.
- Robinson, DB, TY Cai. HDTHe calorimetry 1.0, companion software to our 2015 metal hydride calorimetry paper, archived at osti.gov.