
Melding nanotechnology and medicine, research led by Sandia, the University of New Mexico, and the UNM Cancer Research and Treatment Center has produced an effective strategy to target a cancerous cell using nanoparticles that deliver a mélange of killer drugs into it.
A paper, selected as the cover article of the May issue of Nature Materials and available online April 17, describes silica nanoparticles about 150 nanometers in diameter as honeycombed with cavities that can store large amounts and varieties of drugs.
“The enormous capacity of the nanoporous core, with its high surface area, combined with the improved targeting of an encapsulating lipid bilayer [called a liposome], permit a single ‘protocell’ loaded with a drug cocktail to kill a drug-resistant cancer cell,” says Sandia researcher and UNM professor Jeff Brinker, principal investigator on the research. “That’s a millionfold increase in efficiency over comparable methods employing liposomes alone — without nanoparticles — as drug carriers.”
The nanoparticles, surrounded by cell-like membranes formed from liposomes, together become the combination referred to as a protocell: the membrane seals in the cargo and is modified with molecules (peptides) that bind specifically to receptors overexpressed on the cancer cell surface. The nanoparticles provide stability to the supported membrane and contain and release the therapeutic cargo within the cell.
A current Food and Drug Administration-approved nanoparticle delivery strategy is to use liposomes themselves to contain and deliver the cargo. In a head-to-head comparison of targeted liposomes and protocells with identical membrane and peptide compositions, the paper reports that the greater cargo capacity, stability, and targeting efficacy of protocells leads to orders-of-magnitude greater cytotoxicity directed specifically to human liver cancer cells.
Specialized loading strategies
Another advantage to protocells over liposomes alone, says lead author Carlee Ashley (8621), a Harry S. Truman Fellow at Sandia/California, is that liposomes require specialized loading strategies. “We’ve demonstrated we can simply soak nanoparticles to load them with unique drug combinations needed for personalized medicine. Protocells can also effectively encapsulate potent protein-based toxins, carrying them as well as siRNAs that silence expression of proteins that cancer cells need to survive.”
RNA, the biological messenger that tells cells which proteins to manufacture, is, in this case, used to silence the cellular factory, a way of causing apoptosis or cell death. “Si” is short for “silenced interfering.”
The lipids also serve as a shield that prevents toxic chemotherapy drugs from leaking from the nanoparticle until the protocell binds to and becomes internalized within the cancer cell.
This means that few poisons leak into the system of the human host, should a malignant cell not be located. This cloaking mitigates toxic side effects usually expected from more conventional chemotherapy.
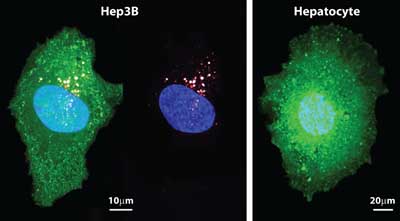
A library of phages — viruses that attack bacteria — was created at UNM’s cancer center by collaborator David Peabody. This permitted researchers to expose the phages to a group of cancerous cells and normal cells, allowing identification of peptides that bind specifically to cancer cells but not normal cells.
“Protocells modified with a targeting peptide that binds to a particular type of cancer exhibit a 10,000-fold greater affinity for that cancer than for non-cancerous cells,” says Carlee.
Jeff adds, “A key feature of our protocell is that the fluid stable supported bilayer allows high-affinity binding with just a few of these peptides overall. This reduces nonspecific binding and immune response.”
The method is being tested on human cancer cells in culture and will shortly be tested in mice at UNM’s nationally accredited cancer center.
Preselecting particles for size
Work still ongoing includes engineering the size of the porous silica particle, which is formed by aerosolizing a precursor solution. The porous nanoparticle fabrication process — called evaporation-induced self-assembly and pioneered in the Brinker lab — produces particles from 50 nanometers to several micrometers in diameter. Particle sizes between 50 and 150 nanometers in diameter are ideal for maximizing circulation and uptake into cancer, so the particles are pre-selected for size before their formation into protocells.
“Their overall dimensions determine how widely they’ll be distributed in the bloodstream,” says Jeff. “We’re altering our synthesis to favor the smaller sizes.”
Also of importance to the circulation time of the particle are its electrical charge and hydrophobicity, which can improve or detract from its ability to remain free of unwanted molecular or energetic entanglements.
Protocells may be ready for testing in humans in as few as five years, researchers estimate.
Jeff is a Sandia Fellow and UNM Regent’s and Distinguished Professor of Chemical and Nuclear Engineering and member of the UNM cancer center. Other institutions involved in the research include the University of California, Davis, and, in Canada, the University of Waterloo.
Funding was provided by the National Cancer Institute, the National Science Foundation, DOE’s Basic Energy Sciences program, the Air Force Office of Scientific Research, and Sandia LDRD.