It may seem a harmless question to ask how molecules of water arrange themselves to cover a surface, but the answer has big consequences. The drag experienced by water flowing past a surface affects the transport of pollutants in the environment. The initial growth of ice crystals on dust is essential to the formation of raindrops.
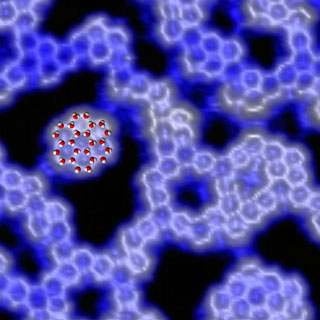
The February issue of Physics Today used this cover image to illustrate work of Peter Feibelman featured in the publication. This scanning tunneling microscope image of water on palladium reveals hexagonal rings of water molecules self-assembled into narrow chains and clusters, as depicted in the superimposed illustration. The arrangement belies the long-held idea of the first water layer as a two-dimensionally continuous molecular mesh, similar to a single “puckered hexagonal” layer of a naturally occurring ice crystal. (Image courtesy of Miquel Salmeron, director of the Materials Sciences Division, Lawrence Berkeley National Laboratory)
In 2001, senior scientist Peter Feibelman (1130) proposed an unexpected solution to a long-standing experimental mystery concerning a one-molecule-thick layer of water on ruthenium: By giving up a hydrogen atom, half the layer’s water molecules find themselves more attracted to the surface. They therefore move closer to it, just as was seen, but not understood, in a 1994 diffraction experiment.
Peter’s technical paper on this work, published in Science (see Lab News, Jan. 25, 2002) has subsequently been referenced more than 200 times — an average, Peter points out with justified pride, of once every two weeks
Now Peter has written, in his usual lucid style, the cover-story article of the current issue of Physics Today, the widely distributed publication of the American Institute of Physics. The article describes research leading up to his seminal paper and the papers following it.
Titled “The first wetting layer on a solid,” the subhead explains that “for decades, researchers imagined that hydrogen bonding imposes a hexagonal, ice-like arrangement on the first water molecules on a solid. Recent theory and experiments argue for a richer view.”
Says Peter, “I had offered an explanation for the odd results of essentially the only quantitative measurement of atom positions on a wetting layer. As I recount in my review, this explanation stirred up a lot of interest, controversy, and further work, including several papers of my own.”
Thereafter, Peter did other work on water-solid interactions, developing theoretical tools to help interpret data from (recently retired Sandian) Jack Houston’s interfacial force microscope, and interpreting atomic-resolution, scanning tunneling microscope pictures of water on metals.
The editors of Physics Today apparently noticed all the activity and emailed to ask Peter who he would recommend to write a review of what was going on. “I gave them several names, but also said I’d be happy to give it a try,” says Peter. “They asked for an outline. I gave them a 2,500 word stream-of-consciousness sample of what I had on my mind. They liked that enough to ask me to write the article.”
The subject might seem abstract. But, as Peter writes in the opening paragraph of his review, “The first layer of water molecules at a surface is the structural template that guides the growth of ice, embodies the boundary condition for water transport, and mediates aqueous interfacial chemistry. It thus determines if rain will fall, how fast pollutants migrate in rock and soil, and governs corrosion, catalysis, and countless other processes.”